NICE Guideline Summaries
Involvement in NICE Guideline Development
The Scientific Committee works with the National Institute for Health and Care Excellence (NICE) to ensure that the views of the Association are represented in the production of Clinical Guidelines, Quality Standards and Diagnostic assessments. Experts are selected by the Scientific Committee on the basis of their knowledge of the subject involved. The level of involvement of the selected member can be is variable and may extend from the reviewing of Draft manuscripts to full membership of the Guideline Development Group.
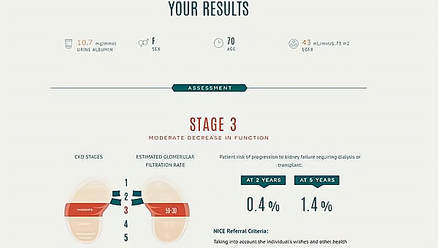
NICE Clinical Guidelines make recommendations to the NHS on treating and caring for people with specific diseases and conditions.
NICE Diagnostics Guidance makes recommendations to the NHS on the efficacy and cost effectiveness of new diagnostic technologies
NICE Quality Standards are a concise set of prioritised statements designed to drive measurable quality improvements within a particular area of health or care.
Guideline Summaries
The Trainees Committee has produced summaries of many published NICE guidelines looking at the affect each guideline has on laboratory services.